Abstract
BACKGROUND: Despite recent improvements with azacitidine (AZA) and venetoclax (VEN), long-term survival for these patients remains short (median overall survival 14.7 months) and rationally designed novel additions to this regimen are being evaluated to improve patient outcomes. CD123 is expressed on the majority of AML blasts and leukemic stem cells while minimally expressed on normal hematopoietic stem cells. Pivekimab sunirine (PVEK, IMGN632) is a first-in-class antibody-drug conjugate (ADC) comprising a high-affinity CD123 antibody, cleavable linker, and an indolinobenzodiazepine pseudodimer (IGN) payload . The novel IGN payload alkylates DNA and causes single strand breaks without crosslinking. IGNs are designed to have high potency against tumor cells, while demonstrating less toxicity to normal marrow progenitors than other DNA-targeting payloads. Both preclinical synergy between PVEK and AZA and/or VEN (Kuruvilla ASH 2020) and initial clinical data in R/R AML (Daver ASH 2021) supported the continued clinical exploration of this triplet. Here we report safety and anti-leukemic activity of PVEK+AZA+VEN from the higher-intensity cohorts and ongoing expansion cohort in patients with R/R AML.
METHODS: In this phase 1b/2 study, eligible patients with CD123-positive R/R AML received PVEK+AZA+VEN in a three-drug escalation over a 28-day cycle: PVEK 0.015 or 0.045 mg/kg day 7, AZA 50 or 75 mg/m2 days 1-7, and VEN 400 mg (or equivalent with azole) for 8, 14, or 21 days. The higher intensity cohorts are defined as patients treated with PVEK on day 7, and either a PVEK dose at 0.045mg/kg or at least 14 days of VEN. Enrollment of relapsed and frontline patients is ongoing in the expansion cohorts of PVEK 0.045 mg/kg day 7, AZA 75 mg/m2 days 1-7, and VEN 400 mg for at least 14 days.
Responses were determined using ELN 2017 criteria (with the addition of CRh and CRp) and a 14-day count recovery window.
RESULTS: As of June 20, 2022, preliminary safety data are available for 71 patients with R/R AML treated in the higher-intensity cohorts as defined above. The median age was 68 years (range, 25-82), 30% had secondary AML, 32% had primary refractory disease, 44% had received prior VEN, 25% had prior allogeneic stem cell transplant, and 54% of the patients were ELN 2017 adverse risk. Fifty-two percent of the patients had received ≥ 2 prior lines of therapy.
The most common treatment-emergent adverse events (TEAE) (all grades [grade 3+ events]) seen in >20% of patients were febrile neutropenia (30% [24%]) and infusion-related reactions (IRRs, 21% [3%]). Pneumonia was reported in 16% of patients [grade 3+ 11%]. Overall, 10% of patients discontinued PVEK due to a TEAE (mostly unrelated to PVEK). Cytopenias and infections were consistent with those observed with HMA+VEN in patients with R/R AML. 30-day mortality was 3%.
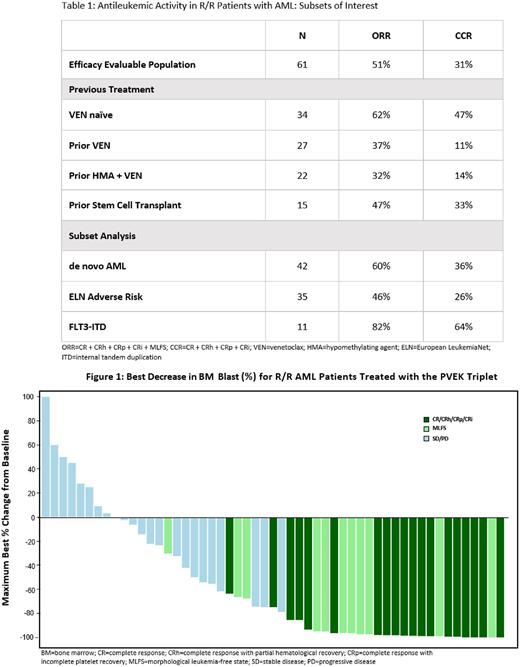
Response data were available for 61 patients with R/R AML. The objective response rate (ORR [CR, CRh, CRp, CRi, MLFS) was 51% with a composite complete remission (CCR [CR, CRh, CRp, CRi]) rate of 31%. VEN-naïve patients had an ORR and CCR of 62% and 47% compared with 37% and 11%, respectively, in patients who had prior VEN exposure. Of note, responses were observed in 9 of 11 patients with FLT3-ITD AML with an ORR of 82% and a CCR of 64%. Table 1 displays response data from sub-groups of interest. Additionally, maximum best change in bone marrow blasts is depicted in Figure 1.
CONCLUSION: With a manageable safety profile in patients with R/R AML, the higher-intensity cohorts of the novel PVEK triplet demonstrated anti-leukemia activity across several difficult-to-treat subsets of patients. Enrollment and follow-up in both relapsed and frontline patients are ongoing (NCT04086264). Additional and updated safety and efficacy data will be presented at ASH.
Disclosures
Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Montesinos:Menarini/Stemline: Consultancy, Research Funding; Gilead: Consultancy, Speakers Bureau; Otsuka: Consultancy; Kura Oncology: Consultancy; ABBVIE: Consultancy, Research Funding, Speakers Bureau; BMS: Research Funding; Novartis: Research Funding; Jazz Pharma: Consultancy, Research Funding, Speakers Bureau; Beigene: Consultancy; Astellas: Consultancy, Speakers Bureau; Incyte: Consultancy; Takeda: Consultancy, Research Funding; Ryvu: Consultancy. Aribi:SeaGen: Consultancy. Marconi:menarini/stemline: Honoraria, Speakers Bureau; astellas: Honoraria; servier: Honoraria; pfizer: Honoraria, Research Funding, Speakers Bureau; abbvie: Research Funding. Altman:Fujifilm: Research Funding; Celgene: Research Funding; Boehringer Ingelheim: Research Funding; Glycomimetics: Other: Data Monitoring Committee; Kartos Therapeutics: Research Funding; Amgen: Research Funding; Aptos: Research Funding; Aprea: Research Funding; Syros: Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Research Funding; Biosight: Membership on an entity's Board of Directors or advisory committees, Other: reumbursement for travel, Research Funding; Abbvie: Honoraria, Research Funding; Astellas: Honoraria, Research Funding; ALX Oncology Inc: Research Funding; Kura Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Loxo: Research Funding. Wang:Abbvie: Consultancy, Honoraria, Other: member of data monitoring committee ; Mana Therapeutics: Consultancy, Honoraria; GlaxoSmithKline: Consultancy, Honoraria, Other: Advisory Board; Rafael Pharmaceuticals: Other: Data Safety Monitoring Committee; Gilead: Consultancy, Honoraria, Other: Advisory Board; Daiichi Sankyo: Consultancy, Honoraria, Other: Advisory Board; PTC Therapeutics: Consultancy, Honoraria, Other: Advisory Board; Macrogenics: Consultancy; Jazz Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Stemline Therapeutics: Consultancy, Honoraria, Other: Advisory Board, Speakers Bureau; Kura Oncology: Consultancy, Honoraria, Other: Advisory Board, Steering Committee, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: Advisory Board; Kite Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Genentech: Consultancy; Takeda: Consultancy, Honoraria, Other: Advisory Board; Pfizer: Consultancy, Honoraria, Other: Advisory Board, Speakers Bureau; Astellas: Consultancy, Honoraria; Dava Oncology: Consultancy, Speakers Bureau; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees. Roboz:MEI Pharma: Consultancy, Research Funding; Mofitt Cancer Center: Research Funding; CTI: Research Funding; Agios: Other: travel, Research Funding; Daiichi Sankyo: Consultancy; Sunesis Pharmaceuticals: Other: Travel and accommodation expenses, Research Funding; MedImmune: Consultancy, Research Funding; Novartis: Consultancy, Other: Travel and accommodation expenses, Research Funding; Jazz: Consultancy, Other: travel; Takeda: Consultancy; Astex Pharmaceuticals: Consultancy, Other: Travel and Accommodation expenses, Research Funding; GlaxoSmithKline: Consultancy; Actinium: Consultancy; Amphivena Therapeutics: Other: Travel and accommodation expenses, Research Funding; Helsinn Therapeutics: Consultancy; Array BioPharma: Other: Travel and accommodation expenses; Roche: Consultancy; Otsuka: Consultancy; Clovis Oncology: Other: Travel and accommodation expenses; Sandoz: Consultancy, Other: Travel and accommodation expenses; Agios: Consultancy, Research Funding; Tensha Therapeutics: Research Funding; Amgen: Consultancy, Other: travel; Bayer: Consultancy, Other: Travel and accommodation expenses; Pfizer: Consultancy, Honoraria, Other: Travel and accommodation expenses; Genentech/Roche: Consultancy, Other: Travel and accommodation expenses; Jasper Therapeutics: Consultancy; Janssen: Consultancy, Other: travel and accommodation expenses, Research Funding; Onconova Therapeutics: Research Funding; Celgene: Consultancy, Other: travel and accommodation expenses, Research Funding; Bristol Myers Squibb: Consultancy; Astellas: Consultancy; Amgen: Consultancy; Eisai: Other: Travel and accommodation expenses; Bristol Myers Squibb: Consultancy; Mesoblast: Consultancy; Celltrion: Consultancy, Other: Travel and accommodation expenses; AbbVie: Consultancy, Other: travel and accommodations, Research Funding; Karyopharm Therapeutics: Research Funding. Gaidano:Astra-Zeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Walter:Amgen: Consultancy, Research Funding; Amphivena Therapeutics, Inc: Current equity holder in publicly-traded company; Aptevo Therapeutics: Consultancy, Research Funding; Arog Pharmaceuticals: Research Funding; Astellas Pharma US, Inc: Consultancy; BioLineRx, LTd: Consultancy, Research Funding; Boston Biomedical, Inc: Consultancy; GSK: Consultancy; Bristol Myers Squibb, Inc: Consultancy; Celgene, Inc: Consultancy, Research Funding; Genentech: Consultancy; ImmunoGen: Research Funding; Janssen Global Services, LLC: Consultancy; Janssen Research and Development: Research Funding; Agios: Consultancy, Research Funding; New Link Genetics: Consultancy; Pfizer, Inc: Consultancy, Research Funding; Race Oncology LTD: Consultancy; Selvita: Research Funding; Stemline Therapeutics: Research Funding; MacroGenics: Consultancy, Research Funding; Kura Oncology: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Kite Pharma, Inc: Consultancy; Kronos Bio, Inc: Consultancy; AbbVie: Consultancy; BerGenBio, ASA: Consultancy; Orum Therapeutics, Inc.: Consultancy. Jeyakumar:Pfizer: Research Funding; Jazz Pharmaceuticals: Research Funding. DeAngelo:Abbvie: Research Funding; Blueprint Medicines Corporation: Consultancy; Incyte: Consultancy; Forty-Seven: Consultancy; Autolus: Consultancy; Agios: Consultancy; Amgen: Consultancy; Glycomimetics: Research Funding; Shire: Consultancy; Takeda: Consultancy; Novartis: Consultancy, Research Funding; Pfizer: Consultancy; Jazz Pharmaceuticals: Consultancy. Erba:Janssen Oncology: Consultancy; Trillium Therapeutics: Consultancy; Covance (Abbvie): Consultancy, Other: Independent Review Committee, Research Funding; Novartis: Consultancy, Research Funding, Speakers Bureau; MacroGenics: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; ImmunoGen: Consultancy, Research Funding; Glycomimetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Celgene: Consultancy, Other, Speakers Bureau; Astellas Pharma: Consultancy; Amgen: Consultancy, Research Funding; Agios: Consultancy, Research Funding, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau; Takeda: Consultancy; Kura Oncology: Consultancy; Forma Therapeutics: Research Funding; Gilead/Forty Seven: Research Funding; PTC therapeutics: Research Funding; ALX Oncology: Research Funding; Pfizer: Consultancy. Todisco:Jazz Pharmacueticals: Consultancy, Other: Advisory Board; Abbvie: Consultancy, Other: Advisory Board; Janssen: Consultancy, Other: Advisory Board. Begna:ImmunoGen: Research Funding; Novartis: Honoraria. Advani:Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Immunogen: Research Funding; OBI: Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Gastaud:Abbvie: Consultancy; Celgene/BMS: Consultancy; Pfizer: Consultancy; GSK: Consultancy. De La Fuente:Celgene: Consultancy, Honoraria, Speakers Bureau; Daiichi Sankyo: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Speakers Bureau. Curti:Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Membership on an entity's Board of Directors or advisory committees. Vyas:Pfizer: Honoraria; Celgene: Honoraria, Research Funding; JAZZ: Honoraria; Abbvie: Honoraria; Astellas: Honoraria; Bristol Myers Squibb: Research Funding; Daiichi Sankyo: Honoraria. Boissel:GILEAD: Honoraria; AMGEN: Honoraria; ARIAD/INCYTE: Honoraria; ASTELLAS: Honoraria; NOVARTIS: Honoraria; SERVIER: Honoraria. Vey:BMS: Honoraria; Novartis: Honoraria, Research Funding; Roche: Honoraria; Janssen: Honoraria; Jazz Pharmaceuticals: Honoraria; Amgen: Honoraria. Recher:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Macrogenics: Honoraria, Membership on an entity's Board of Directors or advisory committees; MaatPharma: Research Funding; Jazz: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria; Incyte: Honoraria; Daiichi Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Platzbecker:Jazz: Honoraria; Geron: Honoraria; Novartis: Honoraria; Takeda: Honoraria; BMS/Celgene: Honoraria; Abbvie: Honoraria; Janssen: Honoraria; Silence Therapeutics: Honoraria. Kapp-Schwoerer:Pfizer Inc.: Consultancy; AbbVie Inc.: Consultancy, Honoraria, Other: Travel support; Jazz Pharmaceuticals: Consultancy, Honoraria, Other: Travel support. Schliemann:Boehringer-Ingelheim: Research Funding; Astrazeneca: Consultancy; Astellas: Consultancy; Abbvie: Consultancy, Other: travel grants; Philogen S.p.A.: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Other: travel grants; Jazz: Consultancy, Research Funding; Novartis: Consultancy; Roche: Consultancy; Pfizer: Consultancy. Konopleva:Kisoji: Consultancy, Honoraria; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Reata Pharmaceuticals: Current equity holder in private company, Patents & Royalties; Eli Lilly: Consultancy, Patents & Royalties, Research Funding; Cellectis: Consultancy, Other: Grant support, Research Funding; Calithera: Other: Grant Support, Research Funding; Ablynx: Other: Grant support, Research Funding; Agios: Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; Rafael Pharmaceutical: Other: grant support, Research Funding; Sanofi: Other: grant support, Research Funding; Novartis: Patents & Royalties, Research Funding; Amgen: Consultancy; Forty-Seven: Consultancy, Honoraria, Other: Grant support; Stemline Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; F. Hoffman La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grant support, Research Funding; Genentech: Consultancy, Other: grant support, Research Funding; AbbVie: Consultancy, Other: grant support, Research Funding. Sallman:Aprea: Membership on an entity's Board of Directors or advisory committees, Research Funding; Nemucore: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Agios: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees; Lixte: Patents & Royalties: LB-100; Kite: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Intellia: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Magenta: Consultancy; Syntrix Pharmaceuticals: Research Funding. Marcucci:Agios: Other: Speaker and advisory scientific board meetings; Novartis: Other: Speaker and advisory scientific board meetings; Abbvie: Other: Speaker and advisory scientific board meetings. Pemmaraju:stemline: Consultancy; abbvie: Consultancy; immunogen: Consultancy; mustangbio: Research Funding; incyte: Consultancy; novartis: Research Funding; pacylex: Consultancy, Research Funding; samus: Research Funding; daiichi sankyo: Research Funding; cellectis: Research Funding; cellularity: Research Funding. Martinelli:Roche: Consultancy; Stemline: Consultancy; Abbvie: Consultancy; Daiichi Sankyo: Consultancy; Jazz Pharmaceuticals: Consultancy; Astellas: Consultancy, Speakers Bureau; Incyte: Consultancy; Pfizer: Consultancy, Speakers Bureau; Celgene/BMS: Consultancy, Speakers Bureau. Kantarjian:Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Research Funding; Jazz Pharmaceuticals: Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; NOVA Research: Honoraria; Amgen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Research Funding; Takeda: Honoraria. Sloss:ImmunoGen: Current Employment. Malcolm:ImmunoGen: Current Employment. Zweidler-McKay:ImmunoGen, Inc.: Current Employment. Sweet:Syntrix Pharmaceuticals: Research Funding; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Curis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; berGenBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Mablytics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AROG: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal